Welcome to the July edition of AOE Compliance Connection, AOE’s monthly newsletter. As we pass the “halfway” mark of 2018, we are hopeful that you are enjoying a busy and productive summer season!
This month’s newsletter features a spotlight on the release of the ACCME’s new website, news about the ABMS/MOC collaboration, board updates for ACCME, ANCC, ACPE and a detailed look at exactly what your LOAs should (and should not) contain.
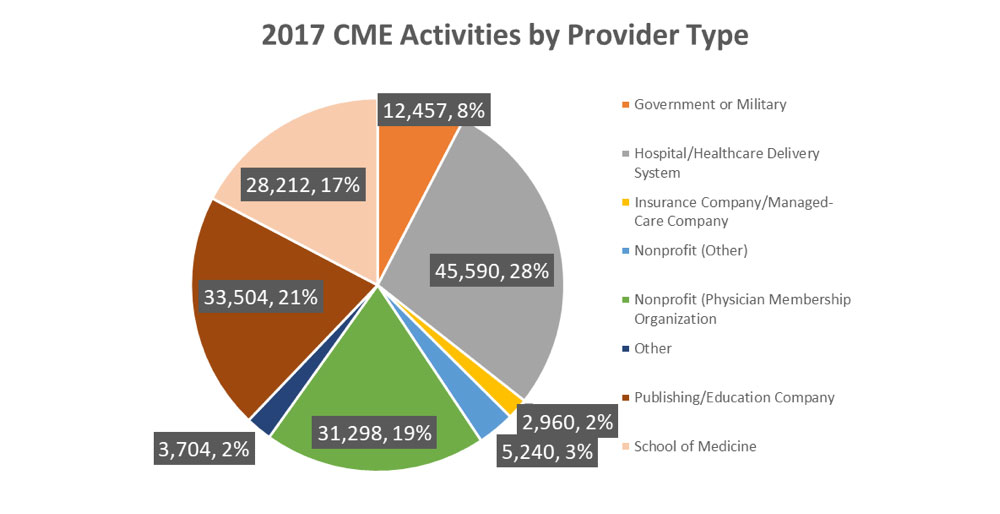
Before we dive in to this month’s edition, we want to bring to your attention the release of the ACCME’s 2017 Annual Report. This report highlights the growth within the CME community, and isolates a few salient points. Data is pulled from more than 1,800 accredited providers (which adds up to nearly 163,000 activities!). 2017 demonstrated an increase in both physician and non-physician participants.
Other statistics and easy-to-digest graphics are available in AOE’s summary of the full report here.
ABMS Continuing Certification Directory
The Continuing Certification of the American Board of Medical Specialties (ABMS) encompasses 24 boards. To be included, a board must first achieve initial Board Certification. The Continuing Certification Directoryis a “central repository of CME activities approved by Member Boards of the ABMS which meet the Lifelong Learning and Self-Assessment requirements of one or more ABMS Member Boards” (www.abms.org). It exists to increase access to CME and Self-Assessment activities, patient safety activities and interdisciplinary competencies. Additionally, the directory increases alignment with other national priorities.
Providers can now submit accredited CME activities via a single submission form. The value of a common submission form is clear – a single pathway for multiple board approvals streamlines the application process. In addition, it advances consistencies in the approval process across multiple boards and enhances the recognition of CME activities that are relevant to multiple specialties. Learners will benefit from the easy access to and identification of activities.
Letters of Agreement: What You Need to Know!
When soliciting commercial support for accredited CME activities, providers must take appropriate steps to ensure compliance with the ACCME Standards for Commercial Support.
Specifically, Standard 3.4 states that, “The terms, conditions, and purposes of commercial support must be documented in a written agreement between the commercial supporter that includes the provider and its educational partner(s). The agreement must include the provider, even if the support is given directly to the provider’s educational partner or a joint provider.”
Commercial support awarded (whether monetary or in-kind), known as an educational grant, or sometimes referred to as an independent medical education grant, must be accompanied by a letter of agreement (LOA) that is signed by the commercial supporter and the accredited provider, at minimum. If the provider is working with an education partner(s) or joint provider, they must be listed in the LOA, but their signatures are optional. The LOA must be signed before launch of the supported activity. Many times, a commercial supporter will have their own LOA, but sometimes it is up to the provider to furnish one. In either instance it is a best practice to review an LOA against a checklist prior to signing. The LOA should include:
- Name and signature of the commercial supporter
- Name and signature of the accredited provider
- Name and signature(s) of any educational partner(s) and/or joint provider(s)
- Dollar amount of the commercial support and/or the nature and amount of “in-kind” support
- The purpose of the commercial support
- Language outlining that the provider has independence regarding educational program decisions and makes all decisions regarding the disposition and disbursement of funds
- Language articulating that the commercial support is solely for supporting the educational initiative
In reviewing an LOA, it is also helpful to note any specific requirements and deadlines so funding is not jeopardized. Often, commercial supporters will require providers to submit a reconciled budget and/or outcomes data. Additionally, sometimes commercial supporters have specific stipulations that preclude funds from being used in a certain manner (e.g. to cover meals).
One final best practice: While LOAs must be reviewed on the front end, prior to signing to ensure compliance, it is also a best practice to review LOAs during reaccreditation, particularly if there has been staffing changes. Documenting any issues found with LOAs and understanding how mistakes came to be aids a provider in determining if management processes need updating or if staff training is necessary.
Accreditation Board Bulletin
Part of the service AOE provides to readers is compliance tips and CME community news for each of the three large boards.
ACCME
The ACCME unveiled a new website this Summer, after receiving input from the CME community. The new site’s navigation is more user-friendly and also mobile device-friendly. The homepage features recent news, upcoming events and CME community information. The principle navigation provides quick access to Accreditation Criteria, Standards for Commercial Support, FAQs and Policies.
Users will find a PARS link in the top banner, so as to easily access it from any page of the website. Also new to the site is a section entitled “Our Stories” where providers are given the opportunity to share examples about how their CME initiatives are making a difference to both patients and to clinicians.
Dr. Graham McMahon, ACCME President and CEO, released a short video highlighting the new website here.
ANCC
Providers accredited by the American Nurses Credentialing Center (ANCC) may begin utilizing the ANCC logo to identify recognized activities. The ANCC Accreditation certification logo may only be used by organizations and programs/courses that are accredited or recognized by the ANCC.
Using the guidelines below, ANCC accredited Providers may add the ANCC logo to letterhead, brochures, and publicity relating to the Program and/or nursing activities within the designated organization.
Guidelines:
- Lettering must be readable – if the words are not legible, the logo must be proportionally resized to increase legibility.
- The logo may be proportionally resized, ensuring the features of the logo are distinguishable; however, the logo may not be altered or modified in any other way.
- The logo may be reproduced in black or purple, in a legible size, without distortion.
- Accreditation logos must be used in conjunction with the accredited organization’s logo, and cannot be used solely. Additionally, accreditation logos may not be larger than the organization’s logo.
- A hyperlink to the ANCC Accreditation Program main landing page must be included on logos used on an accredited organization’s/program’s website –https://www.nursingworld.org/organizational-programs/accreditation/
- If an organization’s accreditation expires, lapses or is revoked/suspended, the organization must immediately stop use of the logo.
- The logo may not be used, reproduced, modified or distributed. Any unauthorized or inappropriate use of the logo is grounds for suspension or revocation of accreditation.
The Accreditation logo should not be used with any goods, products, or services created, offered, or sold that would create the appearance that the ANCC or the Program is providing an endorsement. Logos should only be used as representation of the intent of the Accreditation Program.
For more information on ANCC Provider logos, or to download a logo file, follow this link. When prompted, log-in to the Learning Community with username: accred-provider and password: anccforms.
ACPE
The ASHP/ACPE Accreditation Standards for Pharmacy Technician Education and Training Programs have undergone a thorough revision/update process. The transition will begin this month (July 2018) and will look forward to a full implementation by January 2019.
These Standards were last revised in 2015, and ASHP and ACPE cite a few contributing factors to their latest revisions:
- The recent experience gained by the Pharmacy Technician Accreditation Commission (PTAC) in its accreditation reviews.
- Expansion of the range of pharmacy practice in state laws to include pharmacy technicians.
- Feedback from the 2017 Pharmacy Technician Stakeholder Consensus Conference (PTSCC) about the need for updated entry-level pharmacy technician education and training.
The revised Standards, that now include pharmacy technicians, are intended as public protection by serving as a guide for pharmacy technician education and training program development at the entry- and advanced-levels.
Some changes you’ll see in the revised Standards: Fifteen Standards now appear in three sections, much like the style used by other accrediting bodies. The words “must” and “should” no longer appear, and the Standards instead utilize declarative statements of expectations for compliance. Minimum hour requirements have been edited to reflect education and training needs for entry-level and advanced-level preparation of graduates. Based on PTSCC feedback, the Standards have been divided into entry-level and advanced competencies within one document. Programs may choose if they want to provide only entry-level programs or both entry-level and advanced level programs.
The transition to the final revised Standards begins this month and full implementation is set for January 1, 2019.
- Joint Accreditation Leadership Summit: IPCE Works! Identifying Measures of Success and Evaluating Our Impact
July 29, 2018, Minneapolis, MN
Read More >> - ACCME Accreditation Workshop
August 1-3, 2018, Chicago, IL
Read More >> - ANCC Primary Accreditation Accredited Provider Workshop
August 9, 2018, Silver Spring, MD
Read More >> - ANCC PTAP Introductory Workshop
August 16, 2018, Silver Spring, MD
Read More >> - Beginner CME for MOC: Ask Your Questions Webinar
September 18, 2018
Read More >> - Getting Started in Joint Accreditation
September 28, 2018, Chicago, IL
Read More >>